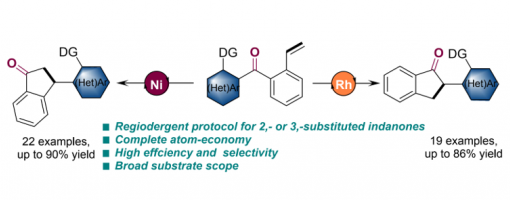
Regiodivergent Access to 2- or 3- Substituted Indanones: Catalyst-Controlled Carboacylation via C–C Bond Activation
Pengcheng Shao,§ Tianyang Yu,§ Hong Lu, Peng-Fei Xu and Hao Wei,*
https://doi.org/10.31635/ccschem.020.202000448
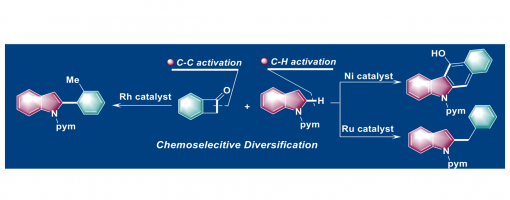
Divergent Coupling of Benzocyclobutenones with Indoles via C−H and C−C Activations
Hong Lu+, Tian-Tian Zhao+, Jin-Hua Bai, Dan Ye, Peng-Fei Xu, and Hao Wei*
https://onlinelibrary.wiley.com/doi/10.1002/anie.202010244
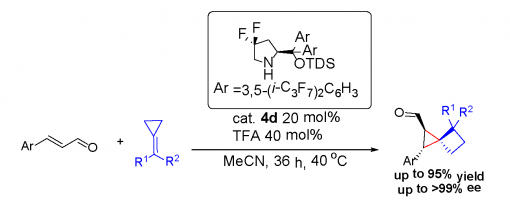
Highly Enantioselective Construction of Strained Spiro[2,3]hexanes through a Michael Addition/Ring Expansion/ Cyclization Cascade
Chuan-Gang Zhao, Zhi-Tao Feng, Guo-Qiang Xu,* Ang Gao, Jing-Wei Chen, Zhu-Yin Wang, and Peng-Fei Xu*
https://doi.org/10.1002/anie.201912834
Enantioselective Synthesis of Spirorhodanine-Pyran Derivatives via Organocatalytic [3 + 3] Annulation Reactions between Pyrazolones and Rhodanine-Derived Ketoesters
Dong-Sheng Ji, Yong-Chun Luo, Xiu-Qin Hu, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.orglett.9b04571
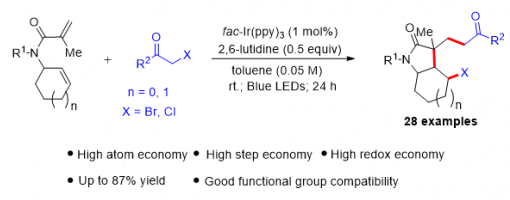
A lutidine-promoted photoredox catalytic atom-transfer radical cyclization reaction for the synthesis of 4-bromo-3,3-dialkyl-octahydroindol-2-ones
Quan-Sheng Zhao, Guo-Qiang Xu, Ji-Tao Xu, Zhu-Yin Wang and Peng-Fei Xu*
https://doi.org/10.1039/C9CC09876C
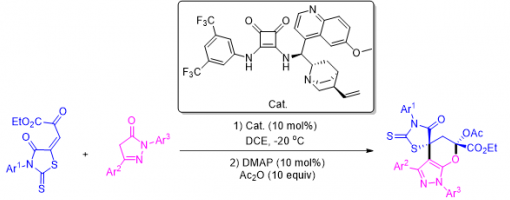
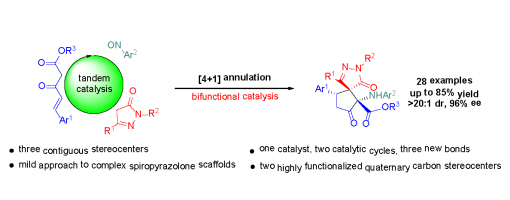
Asymmetric Organocatalytic [4 + 1] Annulations Involving a Polarity Reversal Process: A Tandem Catalytic Approach to Highly Functionalized Spiropyrazolone Derivatives
Chang-Yin Tan, Hong Lu, Jia-Lu Zhang, Jin-Yu Liu, and Peng-Fei Xu*
https://doi.org/10.1021/acs.joc.9b02684
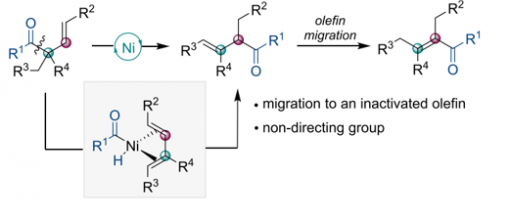
Ni-Catalyzed 1,2-Acyl Migration Reactions Triggered by C−C Bond Activation of Ketones
Cheng Jiang, Hong Lu, Wen-Hua Xu, Jianing Wu, Tian-Yang Yu, Peng-Fei Xu, and Hao Wei*
https://pubs.acs.org/doi/10.1021/acscatal.9b04112
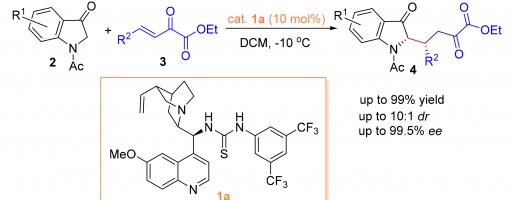
Bifunctional Thioureas Catalyzed Asymmetric Michael Addition of 1-Acetylindolin-3-ones to β,γ-Unsaturated α-Keto Esters
Liu Yaozong, Xu Pengfei, Ma Jianjun, Li Xiaoming, Liang Ruiyuan, Teng Zhijun
https://doi.org/10.6023/cjoc201911027
Abstract
The asymmetric Michael addition of 1-acetylindolin-3-ones to β,γ-unsaturated α-keto esters catalyzed by bifunctional thioureas has been developed. Enantio-pure 2-substituted indolin-3-one derivatives were obtained easily in excellent yields (up to 99%) with good diastereoselectivity (up to 10∶ 1) and excellent enantioselectivities (up to 99.5%), which would be useful for the chiral synthesis of indole-related compounds.