Wan-Lei Yu, Zi-Gang Ren, Wei Ma, Haixue Zheng, Wangsuo Wu and Peng-Fei Xu
Wan-Lei Yu, Zi-Gang Ren, Ke-Xing Ma, Hui-Qing Yang, Jun-Jie Yang, Haixue Zheng, Wangsuo Wu and Peng-Fei Xu
https://doi.org/10.1039/D2SC02291E
Cheng-Lin Pan , Xiang-Xiang Wei , Xu-Gang Zhang , Yong-Wei Xu , Peng-Fei Xu* , Yong-Chun Luo*
https://doi.org/10.1016/j.mineng.2022.107625
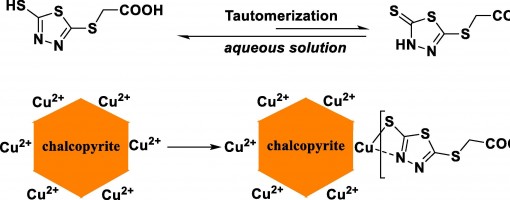
In this work, 2-((5-mercapto-1,3,4-thiadiazol-2-yl)thio)acetic acid (MTAA), a stable and eco-friendly organic small-molecule reagent, was introduced as chalcopyrite depressant for selective flotation separation of molybdenite from chalcopyrite. In the flotation experiments of single mineral and artificially mixed mineral, the recovery of chalcopyrite reduces from 90% to 5% in the pH range of 5 to 10 when MTAA (8 mg/L) is added. The results of IR and zeta potential show that there are two tautomers (thione and thiol) in solid MTAA, while in aqueous solution, MTAA is mainly in the form of thiol and reacts with copper ions. The results of XPS and Tof-SIMS measurements indicate that MTAA may react with copper ions to form a quaternary chelate structure via the S and N atoms. The DFT calculation results also show that MTAA is more likely to react with copper ions than iron ions.
Xu-Gang Zhang , Jie Zhang , Wen-long Ye , Cheng-Lin Pan , Xiang-Xiang Wei , Xiu-Qin Hu , Yong-Chun Luo,* , Peng-Fei Xu*
https://doi.org/10.1016/j.mineng.2022.107602
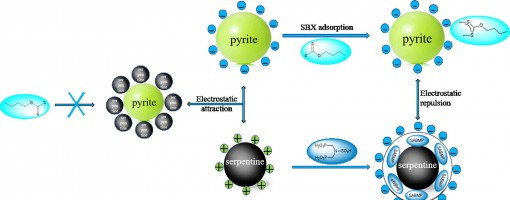
The flotation of pyrite is often depressed by the slime coating of fine serpentine. To eliminate the negative effect of serpentine, N,N-bis(phosphonomethyl)-sulfamic acid (SABMP) was employed as the depressant in this study. The results of micro-flotation experiments showed that the addition of SABMP could significantly reduce the adverse effect of fine serpentine and restore the floatability of pyrite in the pyrite-serpentine system. However, the floatability of pyrite and serpentine had not been obviously reduced in single mineral flotation after the addition of SABMP. The adsorption and ICP results indicated that SABMP could be adsorbed on serpentine surface and simultaneously promote the dissolution of Mg ions on serpentine surface. Further XPS tests demonstrated that SABMP interacted with the Mg species exposed on the surface of serpentine. In addition, it was discovered that the addition of SABMP changed the zeta potential of serpentine from positive to negative at pH 8.5, which eliminated the hetero-coagulation between pyrite and serpentine. The turbidity test further suggested that SABMP could convert the aggregation to the dispersion between pyrite and serpentine. These results demonstrated that SABMP is a potential depressant in the selective flotation separation of pyrite from serpentine.
Sheng-Qiang Guo, Hui-Qing Yang, Yu-Zhen Jiang, Ai-Lian Wang, Guo-Qiang Xu, Yong-Chun Luo, Zhao-Xu Chen, Haixue Zheng and Peng-Fei Xu
https://doi.org/10.1039/D2GC00224H
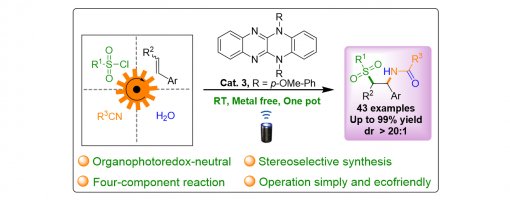
Multicomponent diastereoselective synthesis is still very difficult to be achieved in photoredox catalysis. Here we report a green and reliable strategy for the diastereoselective synthesis of β-amido sulfones through the organophotoredox catalytic fourcomponent radical-polar crossover cascade reactions. This transformation features excellent atom-, step-, redox economy and diastereoselectivity. Moreover, DFT calculation studies are performed to provide some insights into the orign of diastereoselectivity.
Organocatalytic inverse-electron-demand Diels–Alder reaction between 5-alkenyl thiazolones and β,γ-unsaturated carbonyl compounds
Kai-Xuan Yang, Dong-Sheng Ji, Haixue Zheng, Yucheng Gu* and Peng-Fei Xu *
https://doi.org/10.1039/D2CC00457G
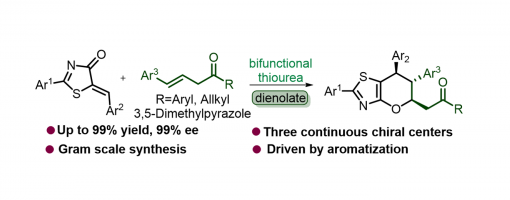
An inverse-electron-demand oxa-Diels–Alder reaction of 5-alkenyl thiazolones with β,γ-unsaturated carbonyl compounds enabled by quinine thiourea was studied, which allows the enantioselective synthesis of a broad range of highly functionalized pyranthiazoles bearing three continuous stereocenters. This protocol is adaptable to a wide scope of substrates and has great potential for scale-up synthesis and facile transformation.
Jie Zhang, Xu-Gang Zhang, Xiang-Xiang Wei, Shao-Yi Cheng, Xiu-Qin Hu, Yong-Chun Luo* Peng-Fei Xu*
https://doi.org/10.1016/j.mineng.2022.107464
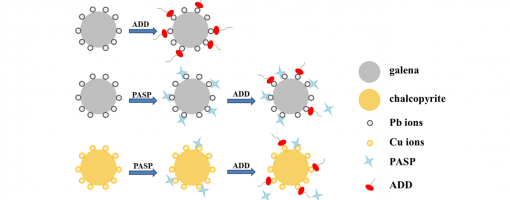
In the flotation separation of copper-lead–zinc complex polymetallic sulphide ores, the separation of chalcopyrite from galena is difficult because both of them have good floatability. Therefore, the development of a green, eco-friendly and selective galena depressant is highly required. In this work, sodium polyaspartate (PASP) was used as a galena depressant in the separation of chalcopyrite from galena. Single mineral flotation experiments showed that PASP could selectively depress galena. With 10 mg/L PASP addition at pH = 10, the recovery of chalcopyrite remained 91.9%, but the recovery of galena decreased to 1.1%. The selective depression mechanism of PASP was studied by collector adsorption measurements, FT-IR and XPS analysis.
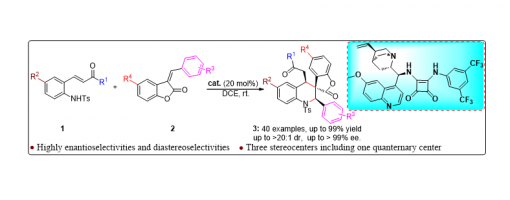
Enantioselective Construction Of Spiro-Tetrahydroquinoline Scaffolds Through Asymmetric Catalytic Cascade Reactions
Jia-Lu Zhang, Rui Ma, Huan-Huan Zhao, Peng-Fei Xu
https://doi.org/10.1039/D2CC00502F
Abstract
An efficient and concise strategy has been successfully developed for merging spiro-tetrahydroquinoline with spiro-benzofuranone into a single new skeleton through asymmetric catalytic cascade reactions catalyzed by quinine-derived chiral bifunctional squaramide organocatalysts. In this approach, differently substituted spiro-tetrahydroquinoline derivatives were smoothly obtained with high yields, and excellent diastereoselectivities and enantioselectivities (up to 99% yield, up to >20 : 1 dr, up to >99% ee, 40 examples) under mild reaction conditions.
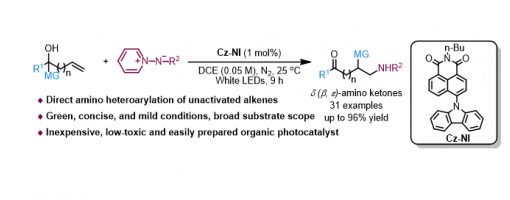
Organic Photoredox Catalytic Amino-Heteroarylation Of Unactivated Olefins To Access Distal Amino Ketones
Ji-Hua Zhang, Teng-Fei Xiao, Zi-Qin Ji, Han-Nan Chen, Pen-Ji Yan, Yong-Chun Luo, Peng-Fei Xu and Guo-Qiang Xu
https://doi.org/10.1039/D1CC07189K
Abstract
Here we describe a metal-free amino-heteroarylation of unactivated olefins via organic photoredox catalysis, providing a concise and efficient approach for the rapid synthesis of various δ (β, ε)-amino ketones under mild conditions. This protocol demonstrates that the new photocatalyst Cz-NI developed by our group has an excellent photoredox catalytic performance. Finally, a series of mechanistic experiments and DFT calculations indicate that this transformation undergoes a photoredox catalytic sequential radical addition/functional group migration process.
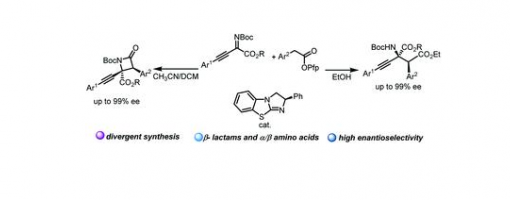
Solvent Directed Chemically Divergent Synthesis Of β-lactams And α-amino Acid Derivatives With Chiral Isothiourea
Dong-Sheng Ji, Hui Liang, Kai-Xuan Yang, Zhi-Tao Feng, Yong-Chun Luo, Guo-Qiang Xu, Yucheng Gu, Peng-Fei Xu
https://doi.org/10.1039/d1sc06127e
Abstract
A protocol for the chemically divergent synthesis of β-lactams and α-amino acid derivatives with isothiourea (ITU) catalysis by switching solvents was developed. The stereospecific Mannich reaction occurring between imine and C(1)-ammonium enolate generated zwitterionic intermediates, which underwent intramolecular lactamization and afforded β-lactam derivatives when DCM and CH3CN were used as solvents. However, when EtOH was used as the solvent, the intermediates underwent an intermolecular esterification reaction, and α-amino acid derivatives were produced. Detailed mechanistic experiments were conducted to prove that these two kinds of products came from the same intermediates. Furthermore, chemically diversified transformations of β-lactam and α-amino acid derivatives were achieved.