Jia-Lu Zhang,†, Wen-Bo He,†, Xiu-Qin Hu, Peng-Fei Xu*
https://doi.org/10.1007/s11426-023-1819-8
http://:https://doi.org/10.1039/D3GC02955G
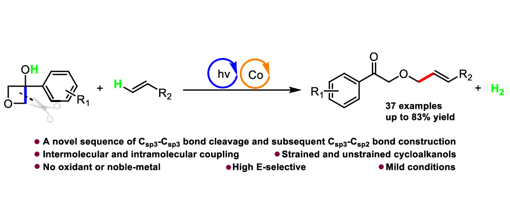
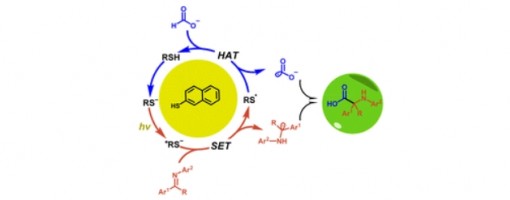
Using a formate salt as a promising hydrogen carrier and one-carbon (C1) source, we have developed a novel and practical method for the preparation of α-amino acid derivatives under mild conditions. In this approach, the photoexcited naphthalene thiolate acts simultaneously as a photoexcited single-electron reductant and a hydrogen atom transfer (HAT) catalyst, enabling efficient metal-free radical–radical cross-coupling of formate with ketimines and aldimines.
https://doi.org/10.1039/D3CC03745B
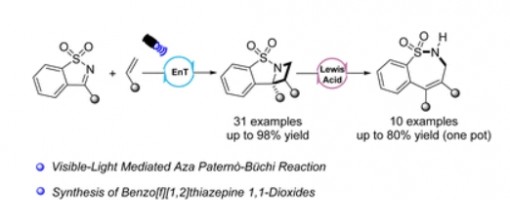
A new two-step, one-pot synthesis of benzo[f][1,2]thiazepine 1,1-dioxides was developed, which contains a visible-light mediated aza Paternò–Büchi reaction of benzo[d]isothiazole 1,1-dioxides with alkenes and a Lewis acid catalyzed ring-expansion of azetidine. In this work, the mechanism of the aza Paternò–Büchi reaction was also investigated.
Yi-Lin Wang,‡ Xin-Xin Lei,‡ Xin-Chen Jin, Xin-Yu Zhang, Peng-Fei Xu* and Yong-Chun Luo*
https://doi.org/10.1039/D3CC02988C
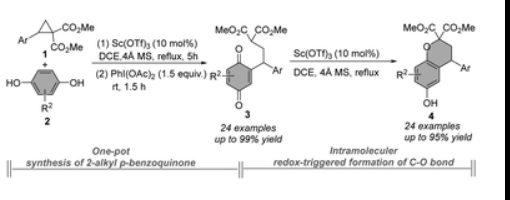
Yong-Chun Luo*, Yang Wang#, Run Shi#, Xu-Gang Zhang, Huan-Huan Zhang and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.3c02068
Pyridinium 1,4-zwitterionic thiolates are usually used to develop ionic annulation reactions. However, radical reactions were rare. We developed a photoredox catalyzed [3 + 2]-annulation reaction of pyridinium 1,4-zwitterionic thiolates with alkenes, disclosed the new reactivity of pyridinium 1,4-zwitterionic thiolate, and provided a new synthetic method for dihydrothiophene.
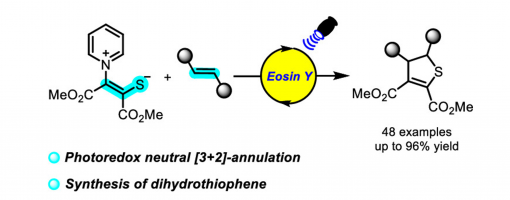
Shao-Xian Shi, Huan-Huan Zhang, Yi-Lin Wang, Lin-Hong Jiang, Peng-Fei Xu* and Yong-Chun Luo*
https://doi.org/10.1021/acs.orglett.3c01695
[2 + 2]-Cycloaddition is the most straightforward approach to the construction of cyclobutanes. In this paper, the intermolecular [2 + 2]-cycloaddition reaction of 3-alkylideneindolin-2-ones with alkenes was achieved. This reaction can be used in the synthesis of 3-spirocyclobutyl oxindoles, polycyclic oxindoles, and late stage modification of some drug molecules.
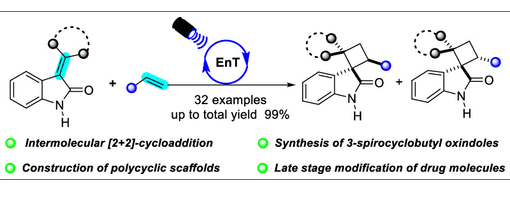
Ma, Rui; Zhang, Jia-Lu; Hu, Xiu-Qin*; Xu, Peng-Fei*
https://doi.org/10.1055/a-2017-4738
Abstract
A series of optically active 3,3′-pyrrolidinyl-dispirooxindole derivatives containing CF3 moiety have been efficiently constructed through asymmetric catalytic cascade reactions catalyzed by cinchona-derived bifunctional squaramide catalyst, bearing four contiguous stereogenic centers and two of which are vicinal spiro-stereocenters. Additionally, a wide range of different substituted products has been achieved with moderate to high yields (up to 99% yield) and excellent stereoselectivities (up to >20:1 dr for all cases and up to 99% ee).
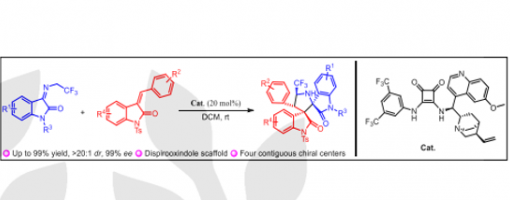
Chen Liu,‡ Han-Nan Chen,‡ Teng-Fei Xiao, Xiu-Qin Hu,* Peng-Fei Xu and Guo-Qiang Xu *
A mild metal-free C–N bond activation strategy for the direct conversion of inert tertiary amines with acyl chlorides into tertiary amides via organic photoredox catalysis is presented. In this protocol, a novel organic photocatalyst (Cz-NI-Ph) that showed excellent catalytic performance during C–N bond cleavage is developed. Moreover, this reaction features green and mild conditions, broad substrate scope, and readily available raw materials.
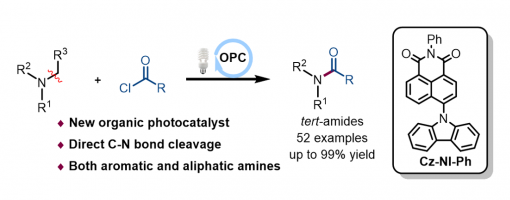
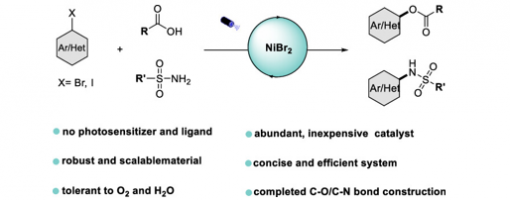
Light-Promoted Nickel-Catalyzed C−O/C−N Coupling of Aryl Halides with Carboxylic Acids and Sulfonamides
Tian-Tian Zhao, Hao-Ni Qin, and Peng-Fei Xu*
Zi-Gang Ren, Wan-Lei Yu, Hai-Xue Zheng, and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.2c03894