1. Asymmetric organocatalysis and synthesis
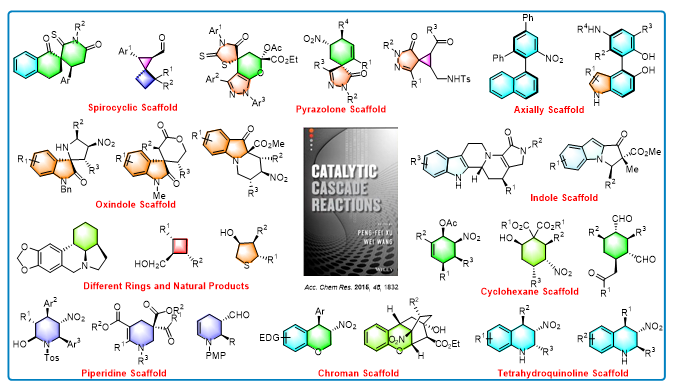
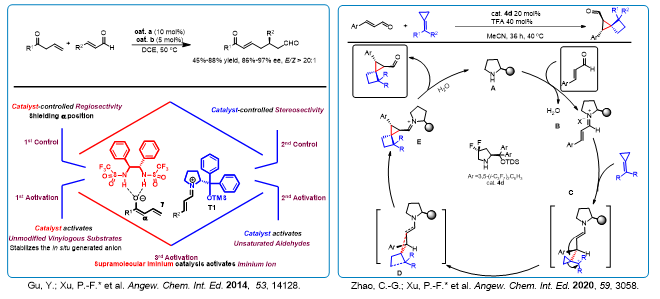
For a long time, the research of our group focuses on exploring the synthetic routes of natural products and drug scaffolds with high efficiency. We have developed many cascade reactions and synthesized a variety of scaffolds with potential biological activities by organocatalysis using chiral amines ,bifunctional thiourea and NHC catalysts. More importantly, in order to solve the practical problems in the field of organocatalysis, two novel concepts were proposed by our group. One is “Asymmetric Organocatalytic Relay Cascades”, the other is “Hydrogen-Bond-mediated Supramolecular Iminum Catalysis”. Based on these concepts and tactics, we have not only significantly reduced the load of catalysts, but also realized the synthesis of epimers controlled by appropriate catalysts.

Small-ring spiro compounds have obvious advantages over ordinary cyclic compounds as bioactive molecules in the medical field. However, the catalytic asymmetric synthesis of small ring spiro compounds is still a great challenge in organic synthesis field. At the beginning of 2020, Xu Pengfei's research group developed a Micheal addition/half pinacol rearrangement/cyclization tandem reaction via asymmetric organocatalysis, and achieved the first synthesis of the high-tension spiro [2,3] hexane skeleton enantioselectively. Three C-C bonds and three continuous chiral centers were constructed using this method in one step, which provides a new synthetic method for the synthesis of small-ring high-tension spiro-skeletons.
2. Catalytic photochemical reactions driven by visible light
As a green, clean and renewableenergy, visible light has been widely used in solar cells and water photodissociation. Different from non-renewable energy sources such as coal and oil, solar energy is the most abundant and accessible energy source in nature. Plants have been transforming carbon dioxide into carbohydrates through photosynthesis for billions of years.
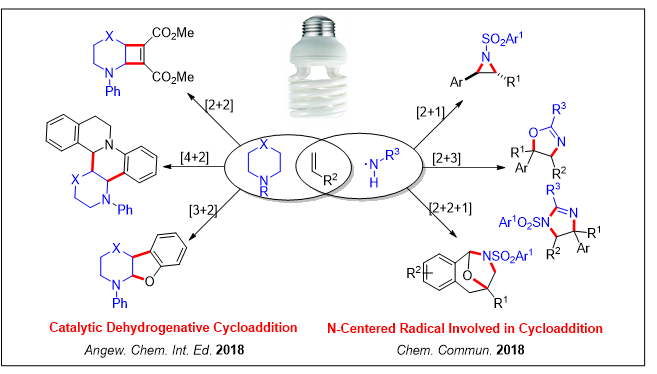
During the past several years, visible light photocatalysis has attracted a great deal of attention from researchers in the field of organic synthesis. Harnessing of energy from visible light as an inexpensive and abundant means to construct complex organic molecules has emerged as a promising method in organic chemistry. The main research works of our group include: 1) dual C-H functionalization of amines for the [2+2] and [4+2] cyclization reactions; 2) dehydrogenative silylation of alkenes for the synthesis of substituted allylsilanes; 3) intramolecular 1,5-H transfer reactions of aryl iodides for the synthesis of oxindole scaffolds and spirocyclic γ-lactams; 4) alkene functionalization for the synthesis of substituted imidazolines, oxazolidines and aziridines.
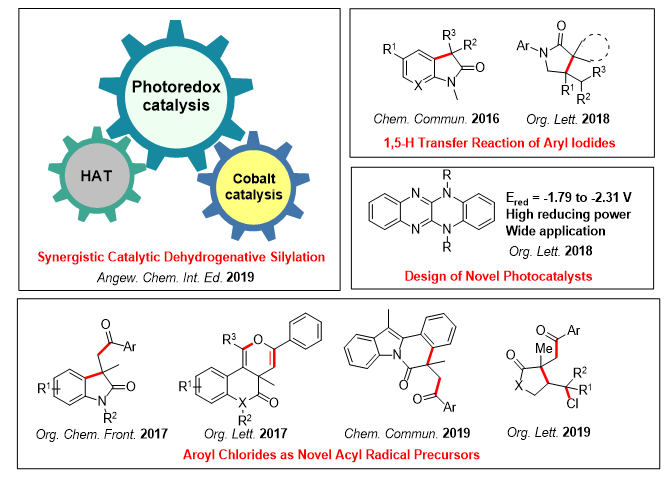
Additionally, Our group is also interested in the design, synthesis, and application of highly reducing organic visible-light photocatalysts, and has generated a variety of radicals from commercially available materials by utilizing visible-light photoredox catalysis.
3. Asymmetric synthesis and methods of α-amino acids

One of our missions is to develop new methodology to synthesize useful building blocks. Amino acids are one of the most important classes of the building blocks of life: they are the structural subunits of proteins, peptides, and many secondary metabolites. Therefore, efficient methods were urgently needed for the synthesis of chiral amino acids. In order to solve this problem, we have developed tricycloiminolactones in our laboratory and used them in the synthesis of several kinds of enantiopure α-amino acids. Facile approaches to several kinds of α-amino acids were developed and successfully applied in the total synthesis of related natural products. The striking advantage of our methodologies is that both enantiomers of α-amino acids can be produced from the same inexpensive and easily accessible chiral sources and the chiral auxiliaries can be recovered and reused. From a synthetic viewpoint, a method which provides both enantiomers of products by using chiral auxiliaries derived from a single chiral source is highly attractive and desirable.
4. Development of novel flotation agents
In the past decades, we are also interested in the development of novel efficient flotation reagents for the copper-nickel sulfide ore in Jinchuan mining area. Several reagents have been successfully used in mineral processing industry and produced economic benefits. For instance, we have designed LJX-1, acting as both a frother and a collector, and a novel activator NS-2. Upon employing the two reagents, the recovery rates of nickel and copper were increased by 1.3% and 0.8%, respectively, averagely extra 530 tons of Nickel and 253 tons of Copper were produced every year. In 2014, wet were awarded the first prize of Scientific and Technological Progress in Gansu Province.