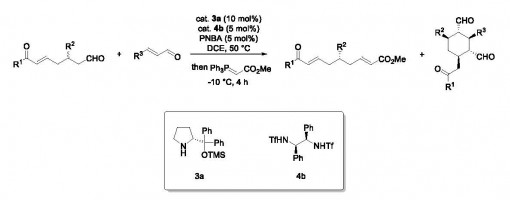
Hong Lu, Yu Zhang, Xiu-Hong Wang, Ran Zhang, Peng-Fei Xu & Hao Wei
https://doi.org/10.1038/s41467-024-48265-6
Developing skeletal editing tools is not a trivial task, and realizing the corresponding single-atom transmutation in a ring system without altering the ring size is even more challenging. Here, we introduce a skeletal editing strategy that enables polycyclic arenols, a highly prevalent motif in bioactive molecules, to be readily converted into N-heteroarenes through carbon–nitrogen transmutation. The reaction features selective nitrogen insertion into the C–C bond of the arenol frameworks by azidative dearomatization and aryl migration, followed by ring-opening, and ring-closing (ANRORC) to achieve carbonto-nitrogen transmutation in the aromatic framework of the arenol. Using widely available arenols as N-heteroarene precursors, this alternative approach allows the streamlined assembly of complex polycyclic heteroaromatics with broad functional group tolerance. Finally, pertinent transformations of the products, including synthesis complex biheteroarene skeletons, were conducted and exhibited significant potential in materials chemistry.
Jie Liu, Hao-Wen Jiang, Xiu-Qin Hu,* and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.4c01186
Considering the ubiquitous presence of pyridine moieties in pharmaceutical compounds, it holds immense value to develop practical and straightforward methodologies for accessing heterocyclic aromatic hydrocarbons. In recent years, N-alkoxypyridinium salts have emerged as convenient radical precursors, enabling the generation of the corresponding alkoxy radicals and pyridine through single-electron transfer. Herein, we present the first report on visible-light-mediated intermolecular alkoxypyridylation of alkenes employing Nalkoxylpyridinium salts as bifunctional reagents with an exceptionally low catalyst loading (0.5 mol %).

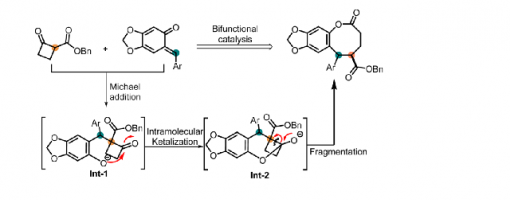
Dong-Sheng Ji,‡Rui Zhang,‡ Xu-Yan Han, Hai-Long Chai, Yucheng Gu, Xiu-Qin Hu and Peng-Fei Xu*
https://doi.org/10.1039/D4QO00148F
Enantioselectively accessing medium-sized ring compounds with multiple stereocenters is still a formidable task due to the unfavorable entropy effect. We have developed a protocol for the enantioselective synthesis of eight-membered lactone derivatives through an organocatalytic Michael/ketalization/fragmentation cascade utilizing ortho-quinone methides and cyclobutanone carbon esters as starting materials. This reaction can be conducted under mild conditions using a wide range of substrates and it exhibits excellent enantioselectivity.
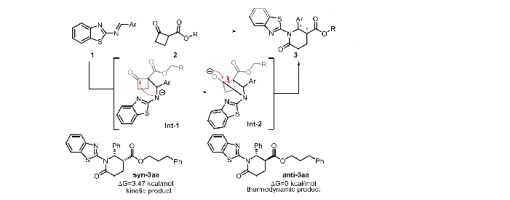
Dong-Sheng Ji,† Rui Zhang,† Xu-Yan Han, Xiu-Qin Hu,* and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.3c03861
A protocol was developed to achieve stereodivergent synthesis of stereoisomers of δ-lactam bearing vicinal chiral centers. Organocatalytic cascade reactions were employed to produce the target products as the kinetic products, which exhibited remarkable enantioselectivities. In the presence of DBU, the kinetic product underwent epimerization to form a thermodynamically more stable diastereomer without loss in enantioselectivity. By simply switching the chiral organocatalyst and its enantiomer, we can efficiently obtain four stereoisomers with high enantioselectivities.
Guo-Qiang Xu*, Wei David Wang*, and Peng-Fei Xu*
https://doi.org/10.1021/jacs.3c06169
Owing to its diverse activation processes including single-electron transfer (SET) and hydrogen-atom transfer (HAT), visible-light photocatalysis has emerged as a sustainable and efficient platform for organic synthesis. These processes provide a powerful avenue for the direct functionalization of C(sp3)–H bonds under mild conditions. Over the past decade, there have been remarkable advances in the enantioselective functionalization of the C(sp3)–H bond via photocatalysis combined with conventional asymmetric catalysis. Herein, we summarize the advances in asymmetric C(sp3)–H functionalization involving visible-light photocatalysis and discuss two main pathways in this emerging field: (a) SET-driven carbocation intermediates are followed by stereospecific nucleophile attacks; and (b) photodriven alkyl radical intermediates are further enantioselectively captured by (i) chiral π-SOMOphile reagents, (ii) stereoselective transition-metal complexes, and (iii) another distinct stereoscopic radical species. We aim to summarize key advances in reaction design, catalyst development, and mechanistic understanding, to provide new insights into this rapidly evolving area of research.

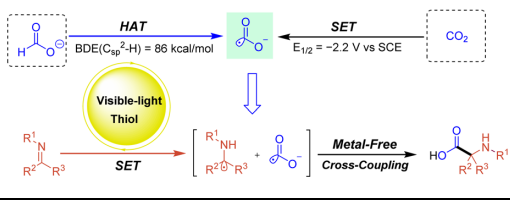
Jia-Lu Zhang1,†, Wen-Bo He1,†, Xiu-Qin Hu1, Peng-Fei Xu1,2,3,*
https://doi.org/10.1007/s11426-023-1819-8
Tian-Tian Zhao,‡a Xu-Gang Zhang,‡a Wen-Bo Hea and Peng-Fei Xu  *ab
*ab
https://doi.org/10.1039/D3GC02955G
Using a formate salt as a promising hydrogen carrier and one-carbon (C1) source, we have developed a novel and practical method for the preparation of α-amino acid derivatives under mild conditions. In this approach, the photoexcited naphthalene thiolate acts simultaneously as a photoexcited single-electron reductant and a hydrogen atom transfer (HAT) catalyst, enabling efficient metal-free radical–radical cross-coupling of formate with ketimines and aldimines.
Synthesis of benzo[f][1,2]thiazepine 1,1-dioxides based on the visible-light-mediated aza Paternò–Büchi reaction of benzo[d]isothiazole 1,1-dioxides with alkenes
Yi-Lin Wang, Peng-Xiang Liu, Huan-Huan Zhang, Peng-Fei Xu and Yong-Chun Luo
https://doi.org/10.1039/D3CC03745B

Yi-Lin Wang,‡ Xin-Xin Lei,‡ Xin-Chen Jin, Xin-Yu Zhang, Peng-Fei Xu* and Yong-Chun Luo*
https://doi.org/10.1039/D3CC02988C

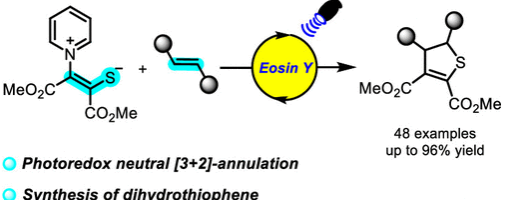
Yong-Chun Luo*, Yang Wang#, Run Shi#, Xu-Gang Zhang, Huan-Huan Zhang and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.3c02068
Pyridinium 1,4-zwitterionic thiolates are usually used to develop ionic annulation reactions. However, radical reactions were rare. We developed a photoredox catalyzed [3 + 2]-annulation reaction of pyridinium 1,4-zwitterionic thiolates with alkenes, disclosed the new reactivity of pyridinium 1,4-zwitterionic thiolate, and provided a new synthetic method for dihydrothiophene.
Shao-Xian Shi, Huan-Huan Zhang, Yi-Lin Wang, Lin-Hong Jiang, Peng-Fei Xu* and Yong-Chun Luo*
https://doi.org/10.1021/acs.orglett.3c01695
Abstract
[2 + 2]-Cycloaddition is the most straightforward approach to the construction of cyclobutanes. In this paper, the intermolecular [2 + 2]-cycloaddition reaction of 3-alkylideneindolin-2-ones with alkenes was achieved. This reaction can be used in the synthesis of 3-spirocyclobutyl oxindoles, polycyclic oxindoles, and late stage modification of some drug molecules.

Enantioselective Synthesis of dispirooxindole Derivatives via asymmetric catalytic cascade reactions
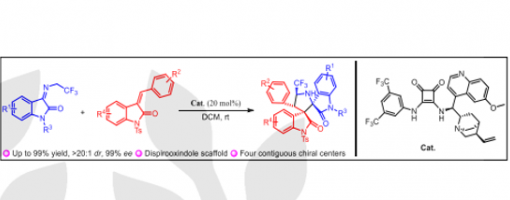
Ma, Rui; Zhang, Jia-Lu; Hu, Xiu-Qin*; Xu, Peng-Fei*
DOI: 10.1055/a-2017-4738
Abstract
A series of optically active 3,3′-pyrrolidinyl-dispirooxindole derivatives containing CF3 moiety have been efficiently constructed through asymmetric catalytic cascade reactions catalyzed by cinchona-derived bifunctional squaramide catalyst, bearing four contiguous stereogenic centers and two of which are vicinal spiro-stereocenters. Additionally, a wide range of different substituted products has been achieved with moderate to high yields (up to 99% yield) and excellent stereoselectivities (up to >20:1 dr for all cases and up to 99% ee).
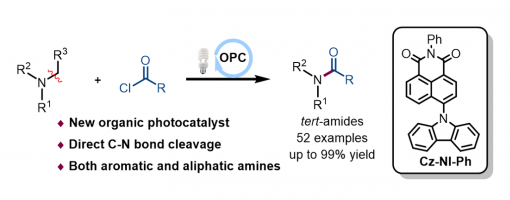
Organic photoredox catalyzed dealkylation/acylation of tertiary amines to access amides
Chen Liu,‡a Han-Nan Chen,‡a Teng-Fei Xiao,a Xiu-Qin Hu,*a Peng-Fei Xu  ab and Guo-Qiang Xu
ab and Guo-Qiang Xu  *a
*a
A mild metal-free C–N bond activation strategy for the direct conversion of inert tertiary amines with acyl chlorides into tertiary amides via organic photoredox catalysis is presented. In this protocol, a novel organic photocatalyst (Cz-NI-Ph) that showed excellent catalytic performance during C–N bond cleavage is developed. Moreover, this reaction features green and mild conditions, broad substrate scope, and readily available raw materials.
Light-Promoted Nickel-Catalyzed C−O/C−N Coupling of Aryl Halides with Carboxylic Acids and Sulfonamides
Tian-Tian Zhao, Hao-Ni Qin, and Peng-Fei Xu*

PCET-Mediated Ring-Opening Alkenylation of Cycloalkanols via Dual Photoredox and Cobalt Catalysis
Zi-Gang Ren, Wan-Lei Yu, Hai-Xue Zheng, and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.2c03894

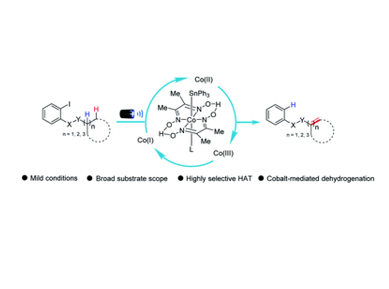
Cobalt-catalyzed chemoselective dehydrogenation through radical translocation under visible light
Wan-Lei Yu,Zi-Gang Ren,Ke-Xing Ma,Hui-Qing Yang, Jun-Jie Yang, Haixue Zheng, Wangsuo Wu and Peng-Fei Xu
https://doi.org/10.1039/D2SC02291E
The transformations that allow the direct removal of hydrogen from their corresponding saturated counterparts by the dehydrogenative strategy are a dream reaction that has remained largely underexplored. In this report, a straightforward and robust cobaloxime-catalyzed photochemical dehydrogenation strategy via intramolecular HAT is described for the first time. The reaction proceeds through an intramolecular radical translocation followed by the cobalt assisted dehydrogenation without needing any other external photosensitizers, noble-metals or oxidants. With this approach, a series of valuable unsaturated compounds such as α,β-unsaturated amides, enamides and allylic and homoallylic sulfonamides were obtained in moderate to excellent yields with good chemo- and regioselectivities, and the synthetic versatility was demonstrated by a range of transformations. And mechanistic studies of the method are discussed.
https://doi.org/10.1039/D2GC02084J
Direct C–H/N–H dehydrogenative coupling is a promising yet thermodynamically unfavorable transformation in the absence of a sacrificial hydrogen acceptor. Herein, a conceptually novel oxidant-free dehydrogenative amination of alkenes through a synergistic photoredox and cobalt catalysis with H2 evolution has been achieved. With this approach, a wide range of five-membered N-heterocycles were synthesized with excellent atom-economy. The green system will address the challenges that are sensitive to traditionally oxidative conditions. Furthermore, the scope and mechanistic details of the method are discussed.

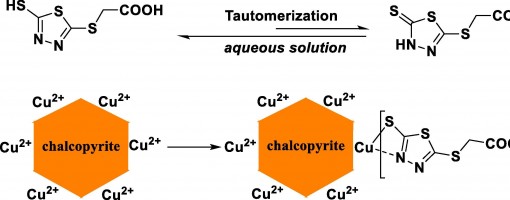
Cheng-Lin Pan , Xiang-Xiang Wei , Xu-Gang Zhang , Yong-Wei Xu , Peng-Fei Xu* , Yong-Chun Luo*
https://doi.org/10.1016/j.mineng.2022.107625
In this work, 2-((5-mercapto-1,3,4-thiadiazol-2-yl)thio)acetic acid (MTAA), a stable and eco-friendly organic small-molecule reagent, was introduced as chalcopyrite depressant for selective flotation separation of molybdenite from chalcopyrite. In the flotation experiments of single mineral and artificially mixed mineral, the recovery of chalcopyrite reduces from 90% to 5% in the pH range of 5 to 10 when MTAA (8 mg/L) is added. The results of IR and zeta potential show that there are two tautomers (thione and thiol) in solid MTAA, while in aqueous solution, MTAA is mainly in the form of thiol and reacts with copper ions. The results of XPS and Tof-SIMS measurements indicate that MTAA may react with copper ions to form a quaternary chelate structure via the S and N atoms. The DFT calculation results also show that MTAA is more likely to react with copper ions than iron ions.
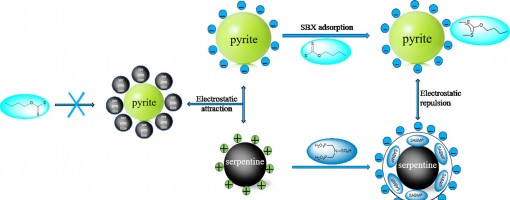
Xu-Gang Zhang , Jie Zhang , Wen-long Ye , Cheng-Lin Pan , Xiang-Xiang Wei , Xiu-Qin Hu , Yong-Chun Luo,* , Peng-Fei Xu*
https://doi.org/10.1016/j.mineng.2022.107602
The flotation of pyrite is often depressed by the slime coating of fine serpentine. To eliminate the negative effect of serpentine, N,N-bis(phosphonomethyl)-sulfamic acid (SABMP) was employed as the depressant in this study. The results of micro-flotation experiments showed that the addition of SABMP could significantly reduce the adverse effect of fine serpentine and restore the floatability of pyrite in the pyrite-serpentine system. However, the floatability of pyrite and serpentine had not been obviously reduced in single mineral flotation after the addition of SABMP. The adsorption and ICP results indicated that SABMP could be adsorbed on serpentine surface and simultaneously promote the dissolution of Mg ions on serpentine surface. Further XPS tests demonstrated that SABMP interacted with the Mg species exposed on the surface of serpentine. In addition, it was discovered that the addition of SABMP changed the zeta potential of serpentine from positive to negative at pH 8.5, which eliminated the hetero-coagulation between pyrite and serpentine. The turbidity test further suggested that SABMP could convert the aggregation to the dispersion between pyrite and serpentine. These results demonstrated that SABMP is a potential depressant in the selective flotation separation of pyrite from serpentine.
Sheng-Qiang Guo, Hui-Qing Yang, Yu-Zhen Jiang, Ai-Lian Wang, Guo-Qiang Xu, Yong-Chun Luo, Zhao-Xu Chen, Haixue Zheng and Peng-Fei Xu
https://doi.org/10.1039/D2GC00224H
Multicomponent diastereoselective synthesis is still very difficult to be achieved in photoredox catalysis. Here we report a green and reliable strategy for the diastereoselective synthesis of β-amido sulfones through the organophotoredox catalytic fourcomponent radical-polar crossover cascade reactions. This transformation features excellent atom-, step-, redox economy and diastereoselectivity. Moreover, DFT calculation studies are performed to provide some insights into the orign of diastereoselectivity.
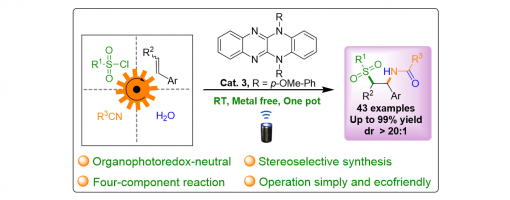
Kai-Xuan Yang, Dong-Sheng Ji, Haixue Zheng, Yucheng Gu* and Peng-Fei Xu *
https://doi.org/10.1039/D2CC00457G
An inverse-electron-demand oxa-Diels–Alder reaction of 5-alkenyl thiazolones with β,γ-unsaturated carbonyl compounds enabled by quinine thiourea was studied, which allows the enantioselective synthesis of a broad range of highly functionalized pyranthiazoles bearing three continuous stereocenters. This protocol is adaptable to a wide scope of substrates and has great potential for scale-up synthesis and facile transformation.
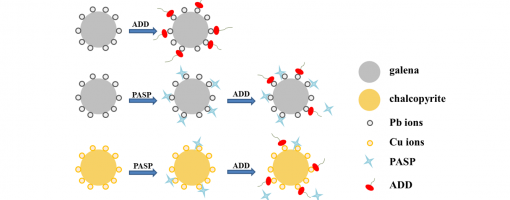
Jie Zhang, Xu-Gang Zhang, Xiang-Xiang Wei, Shao-Yi Cheng, Xiu-Qin Hu, Yong-Chun Luo* Peng-Fei Xu*
https://doi.org/10.1016/j.mineng.2022.107464
In the flotation separation of copper-lead–zinc complex polymetallic sulphide ores, the separation of chalcopyrite from galena is difficult because both of them have good floatability. Therefore, the development of a green, eco-friendly and selective galena depressant is highly required. In this work, sodium polyaspartate (PASP) was used as a galena depressant in the separation of chalcopyrite from galena. Single mineral flotation experiments showed that PASP could selectively depress galena. With 10 mg/L PASP addition at pH = 10, the recovery of chalcopyrite remained 91.9%, but the recovery of galena decreased to 1.1%. The selective depression mechanism of PASP was studied by collector adsorption measurements, FT-IR and XPS analysis.
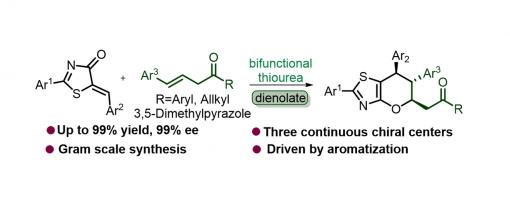
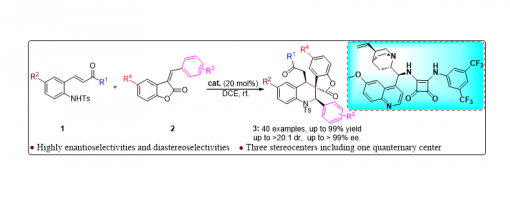
Enantioselective Construction Of Spiro-Tetrahydroquinoline Scaffolds Through Asymmetric Catalytic Cascade Reactions
Jia-Lu Zhang, Rui Ma, Huan-Huan Zhao, Peng-Fei Xu
https://doi.org/10.1039/D2CC00502F
Abstract
An efficient and concise strategy has been successfully developed for merging spiro-tetrahydroquinoline with spiro-benzofuranone into a single new skeleton through asymmetric catalytic cascade reactions catalyzed by quinine-derived chiral bifunctional squaramide organocatalysts. In this approach, differently substituted spiro-tetrahydroquinoline derivatives were smoothly obtained with high yields, and excellent diastereoselectivities and enantioselectivities (up to 99% yield, up to >20 : 1 dr, up to >99% ee, 40 examples) under mild reaction conditions.
Organic Photoredox Catalytic Amino-Heteroarylation Of Unactivated Olefins To Access Distal Amino Ketones
Ji-Hua Zhang, Teng-Fei Xiao, Zi-Qin Ji, Han-Nan Chen, Pen-Ji Yan, Yong-Chun Luo, Peng-Fei Xu and Guo-Qiang Xu
https://doi.org/10.1039/D1CC07189K
Abstract
Here we describe a metal-free amino-heteroarylation of unactivated olefins via organic photoredox catalysis, providing a concise and efficient approach for the rapid synthesis of various δ (β, ε)-amino ketones under mild conditions. This protocol demonstrates that the new photocatalyst Cz-NI developed by our group has an excellent photoredox catalytic performance. Finally, a series of mechanistic experiments and DFT calculations indicate that this transformation undergoes a photoredox catalytic sequential radical addition/functional group migration process.

Solvent Directed Chemically Divergent Synthesis Of β-lactams And α-amino Acid Derivatives With Chiral Isothiourea
Dong-Sheng Ji, Hui Liang, Kai-Xuan Yang, Zhi-Tao Feng, Yong-Chun Luo, Guo-Qiang Xu, Yucheng Gu, Peng-Fei Xu
https://doi.org/10.1039/d1sc06127e
Abstract
A protocol for the chemically divergent synthesis of β-lactams and α-amino acid derivatives with isothiourea (ITU) catalysis by switching solvents was developed. The stereospecific Mannich reaction occurring between imine and C(1)-ammonium enolate generated zwitterionic intermediates, which underwent intramolecular lactamization and afforded β-lactam derivatives when DCM and CH3CN were used as solvents. However, when EtOH was used as the solvent, the intermediates underwent an intermolecular esterification reaction, and α-amino acid derivatives were produced. Detailed mechanistic experiments were conducted to prove that these two kinds of products came from the same intermediates. Furthermore, chemically diversified transformations of β-lactam and α-amino acid derivatives were achieved.

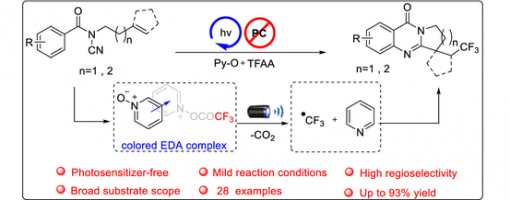
A Photosensitizer–Free Radical Cascade for Synthesizing CF3-Containing Polycyclic Quinazolinones with Visible Light
Qiang Hu, Wan-Lei Yu, Yong-Chun Luo, Xiu-Qin Hu*, and Peng-Fei Xu*
https://doi.org/10.1021/acs.joc.1c02889
Abstract
Herein, we report an efficient photoinduced radical tandem trifluoromethylation/cyclization reaction of N-cyanamide alkenes for the synthesis of functionalized quinazolinones. Importantly, the reaction is carried out under mild conditions without any additional photosensitizer, metal, or extra additives. A series of trifluoromethyl quinazolinones were prepared efficiently with good yields and excellent functional group tolerance. Preliminary mechanistic experiments were conducted to indicate that the transformation proceeds via a possible mechanism involving photoexcited EDA complex and chain propagation.
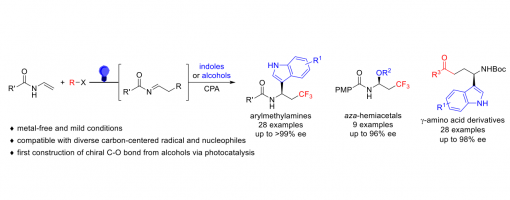
Metal-Free, Visible-Light Induced Enantioselective Three-Component Dicarbofunctionalization And Oxytrifluoromethylation Of Enamines Via Chiral Phosphoric Acid Catalysis
Hui Liang, Dong-Sheng Ji, Guo-Qiang Xu, Yong-Chun Luo, Hai-Xue Zheng* and Peng-Fei Xu *
https://doi.org/10.1039/D1SC06613G
Abstract
Using diverse carbon-centered radical precursors and electron-rich (hetero)aromatics and alcohols as nucleophiles, a visible-light driven chiral phosphoric acid (CPA) catalyzed asymmetric intermolecular, three-component radical-initiated dicarbofunctionalization and oxytrifluoromethylation of enamines was developed, which provides a straightforward access to chiral chiral arylmethylamines aza-hemiacetals and γ-amino acid derivatives with excellent enantioselectivity. As far as we know, this is the first example of constructing a chiral C-O bond using simple alcohols via visible-light photocatalysis. Chiral phosphoric acid played multiple roles in the reaction, including controlling the reaction stereoselectivity and promoting the generation of radical intermediates by activating Togni’s reagent. Mechanistic studies also suggested the importance of the N-H bond of the enamine and indole for the reactions.
Selective Flotation Separation Of Chalcopyrite From Talc By A Novel Depressant: N-methylene Phosphonic Chitosan
Wen-Long Ye, Xu-Gang Zhang, Cheng-Lin Pan, Xiu-Qin Hu*, Yong-Chun Luo*, Peng-Fei Xu*
https://doi.org/10.1016/j.mineng.2021.107367
In this work, we investigated the flotation performance and selective adsorption mechanism of a chitosan derivative NMPC (N-methylene phosphonic chitosan) as an novel talc depressant in the flotation separation of chalcopyrite from talc. The single mineral flotation experiments demonstrated that without the talc depressant, both chalcopyrite and talc showed excellent floatability, thus the separation was difficult in the pH range of 3–11. When NMPC was added, the recovery of talc was reduced drastically but the recovery of chalcopyrite decreased slightly in the pH range of 3–9 at a concentration of 10 mg/L in pulp. Artificial mixed mineral flotation experiments also demonstrated that the effective separation of talc and chalcopyrite could be achieved when NMPC was employed as the depressant. The results showed that better grade and recovery were obtained in different proportions of chalcopyrite and talc, which meant the NMPC is better than CMC, Arabic gum in flotation separation of talc and chalcopyrite. Analysis of adsorption tests, FT-IR measurements, and XPS measurements suggested that NMPC was selectively adsorbed on the talc surface by physical adsorption which might result from the hydrogen bonding and hydrophobic interaction between NMPC and talc. Moreover, the sedimentation experiments disclosed that NMPC plays an important role in the flocculation and settling of fine talc.
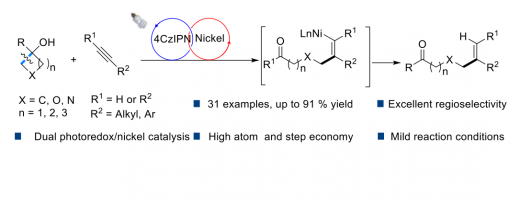
Photoredox/Nickel Dual Catalyzed Stereospecific Synthesis Of Distal Alkenyl Ketones
Tian-Tian Zhao, Wan-Lei Yu, Zhi-Tao Feng, Hao-Ni Qin, Hai-xue Zheng* and Peng-Fei Xu*
https://doi.org/10.1039/D1CC06566A
The selective C–C bond deconstruction/refunctionalization via a photoredox/nickel dual-catalyzed hydroalkylation of alkynes is developed under mild reaction conditions. In this protocol, a broad range of alkyl- and aryl-alkynes could react smoothly with cycloalkanols, affording the corresponding distal and site-specific vinyl-substituted ketones with high yields and excellent regioselectivities. Moreover, DFT calculations verified that the electron-rich behavior of aromatics and weak Brønsted bases have a common effect on the photocatalytic oxidant ring-opening of cyclobutanols.
Organic Photoredox Catalytic α-C(sp3)–H Phosphorylation of Saturated Aza-Heterocycles
Ming-Jun Yi,‡ Teng-Fei Xiao,‡ Wen-Hui Li, Yi-Fan Zhang, Pen-Ji Yan, Baoxin Zhang, Peng-Fei Xu and Guo-Qiang Xu
https://doi.org/10.1039/D1CC05767G
Abstract
A metal-free C(sp3)–H phosphorylation of saturated aza-heterocycles via the merger of organic photoredox and Brønsted acid catalysis was established under mild conditions. This protocol provided a straightforward and economic access to a variety of valuable α-phosphoryl cyclic amines by using commercially available diarylphosphine oxide reagents. In addition, the D-A fluorescent molecule DCQ was firstly used as a photocatalyst and exhibited excellent photoredox catalytic efficiency in this transformation. A series of mechanistic experiments and DFT calculations demonstrated that this transformation underwent a sequential visible-light photoredox catalytic oxidation/nucleophilic addition process.

Divergent Ritter-type amination via photoredox catalytic four-component radical-polar crossover reactions
Sheng-Qiang Guo, Hui-Qing Yang, Ai-Lian Wang, Yu-Zhen Jiang, Guo-Qiang Xu, Yong-Chun Luo,* Zhao-Xu Chen and Peng-Fei Xu*
https://doi.org/10.1039/D1GC03048E
A green and practical divergent Ritter-type amination via a photoredox catalytic four-component radical-polar crossover process was developed. This versatile protocol presents a modular and powerful strategy to access functionalized β-sulfonyl imides and imines under mild conditions from readily available and stable substrates with high atom-, step- and redox economy. We manipulated carboxylic acids and benzotriazoles to precisely attack the nitrilium ion instead of the carbocation, thus preventing many complicated side reactions of the photocatalytic reactions. Furthermore, the synthetic utility of this divergent Ritter reaction is further demonstrated by the late-stage modification of pharmaceutical and natural product derivatives.

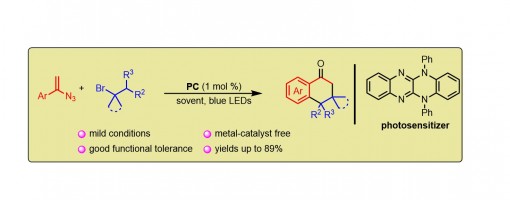
Visible-Light-Promoted Multi-step Tandem Reaction of Vinyl Azides toward the Formation of 1-Tetralones
Meng-Jie Jiao, Qiang Hu, Xiu-Qin Hu* and Peng-Fei Xu*
https://doi.org/10.1021/acs.joc.1c02261
ABSTRACT
A visible-light-driven multi-step tandem reaction between vinyl azides and alkyl bromides has been developed leading to the formation of tetralone skeletons under mild conditions, which can be easily scaled up to the gram scale. Various 1-tetralone derivatives are synthesized and transformed into desired products in good to high yields.
Dehydrogenation/(3+2) Cycloaddition of Saturated Aza-Heterocycles via Merging Organic Photoredox and Lewis Acid Catalysis
Teng-Fei Xiao, Yi-Fan Zhang, Wen-Tao Hou, Pen-Ji Yan, Jun Hai, Peng-Fei Xu*, and Guo-Qiang Xu*https://doi.org/10.1021/acs.orglett.1c03431
Abstract
Herein, we report a photoinduced dehydrogenation/(3+2) cycloaddition reaction by merging organic photoredox and Lewis acid catalysis, providing a straightforward and efficient approach for directly installing a benzofuran skeleton on the saturated aza-heterocycles. In this protocol, we also describe a novel organic photocatalyst (t-Bu-DCQ) with the advantages of a wider redox potential, easy synthesis, and a low price. Furthermore, the stepwise activation mechanism of dual C(sp3)–H bonds was demonstrated by a series of experimental and computational studies.

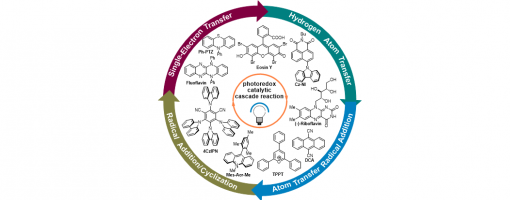
Visible-light organic photoredox catalytic cascade reaction
Guo-Qiang Xu* and Peng-Fei Xu*
https://doi.org/10.1039/D1CC04883J
Abstract
Over the past years, impressive progress has been made on the development of organic photoredox catalytic cascade reactions without the participation of expensive and toxic transition-metals under visible light irradiation. These transformations highly depend on the in situ generation of various radical species in the photoredox catalytic cycles. Numerous chemically and biomedically valuable building blocks have been synthesized through this efficient and sustainable protocol. In this review, we highlight the recent progress in this blooming area by presenting a series of new catalytic cascade reactions mediated by organic photoredox catalysts and describe their mechanisms and applications which have appeared in the recent literature.
Enantioselective Construction of Polycyclic Indazole Skeletons Bearing Five Consecutive Chiral Centers through an Asymmetric Triple-Reaction Sequence
Jia-Lu Zhang, Wen-Long Ye, Jie Zhang, Xiu-Qin Hu,* and Peng-Fei Xu*
DOI: 10.1021/acs.orglett.1c01559
ABSTRACT: A novel approach for the asymmetric construction of polycyclic indazole skeletons via enamine−imine activation and PCET activation was developed by merging organocatalysis with photocatalysis through an asymmetric triple-reaction sequence. In this process, five C−X bonds and five consecutive chiral centers were efficiently constructed. Differently substituted polycyclic indazole deriatives were successfully constructed with satisfactory results under mild conditions.

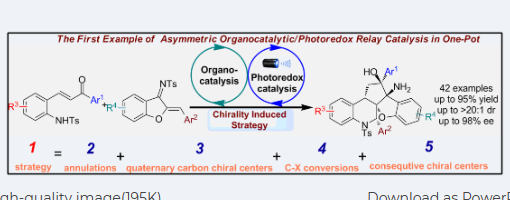
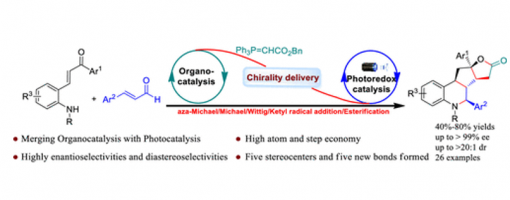
One-Pot Enantioselective Construction of Polycyclic Tetrahydroquinoline Scaffolds through Asymmetric Organo/Photoredox Catalysis via Triple-Reaction Sequence
Jia-Lu Zhang, Jin-Yu Liu, Guo-Qiang Xu, Yong-Chun Luo, Hong Lu, Chang-Yin Tan, Xiu-Qin Hu*, and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.1c00712
Abstract
A novel one-pot triple-reaction strategy for the asymmetric construction of polycyclic skeletons with multiple consecutive chiral centers through aza-Michael/Michael/Wittig/ketyl radical addition/esterification processes is reported. A wide range of polycyclic tetrahydroquinoline derivatives were smoothly obtained from easily available starting materials with good results (up to 80% yield, >20:1 dr, >99% ee) under mild conditions. In this transformation, five chemical bonds and five consecutive chiral centers were successively formed.
Metal-Free α-C(sp3)–H Aroylation of Amines via a Photoredox Catalytic Radical–Radical Cross-Coupling Process
Guo-Qiang Xu*, Teng-Fei Xiao, Guo-Xuan Feng, Chen Liu, Baoxin Zhang, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.orglett.1c00226
Abstract
Here we describe an unprecedented metal-free C(sp3)–H aroylation of amines via visible-light photoredox catalysis, which provides a straightforward route for the construction of a useful α-amino aryl ketone skeleton. Additionally, a number of selected products exhibit good biological activity for protecting PC12 cell damage, which shows that this skeleton has the potential to become a new neuroprotective agent. Finally, a series of mechanism experiments indicate that this transformation undergoes a photoredox catalytic radical–radical cross-coupling pathway.

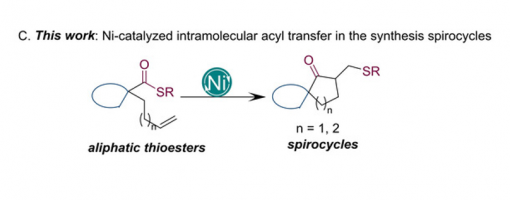
Synthesis of Spirocycles via Ni‐Catalyzed Intramolecular Coupling of Thioesters and Olefins
Wenfei Liu, Dr. Wenhua Xu, Juanjuan Wang, Dr. Hong Lu, Prof. Dr. Peng‐Fei Xu, Prof. Dr. Hao Wei
https://doi.org/10.1002/chem.202100390
Abstract
A nickel‐catalyzed intramolecular coupling of thioesters and olefins has been developed for the efficient synthesis of spirocycles, a privileged scaffold commonly found in natural products. This transformation is characterized by the simultaneous transfer of both acyl and thiol moieties to the alkene, with the suppression of decarbonylation and β‐hydrogen elimination. Initial mechanistic investigations are consistent with an oxidative addition/olefin insertion/reductive elimination mechanism. The incorporated methylene sulfide substituent can undergo a variety of further reactions to increase molecular diversity and complexity. These results demonstrate that thioester derivatives can be used as powerful building blocks for the assembly of complex scaffolds.
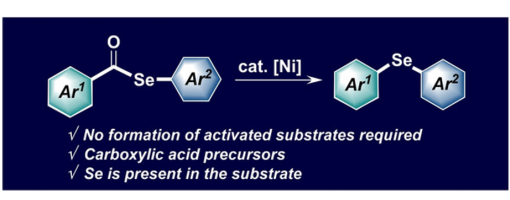
Nickel‐Catalyzed Intramolecular Decarbonylative Coupling of Aryl Selenol Esters
Jin‐Hua Bai, Xiu‐Juan Qi, Wei Sun, Tian‐Yang Yu, Peng‐Fei Xu
https://doi.org/10.1002/adsc.202001611
Absrtact
This report describes a method for Ni‐catalyzed intramolecular decarbonylative coupling, which enables the conversion of areneselenol esters to diaryl selenides. The inexpensive and readily available catalyst can be employed under mild reaction conditions for the construction of structurally diverse diaryl selenides, including heterocyclic and natural product derivatives.
Sc(OTf)3 catalyzed [3 + 2]-annulation reaction of donor–acceptor aziridines with methylene exo-glycals: synthesis of chiral carbohydrate-spiro-heterocycles
Jie-Hui Zhang, Cheng-Lin Pan, Huan-Huan Zhang, Peng-Fei Xu* and Yong-Chun Luo*
https://doi.org/10.1039/D1QO00228G
A Sc(OTf)3 catalyzed [3 + 2]-annulation reaction between donor–acceptor aziridines and several exo-glycals has been developed. Using this method, some exo-glycals derived from D-ribose, D-galactose and uridine can be converted into carbohydrate-spiro-heterocycles easily in good yields. Simple derivatization of the products gives proline analogues and polycyclic compounds. Moreover, this reaction disclosed the stereochemistry characteristic of the reaction between racemic D–A aziridines and chiral enolethers.

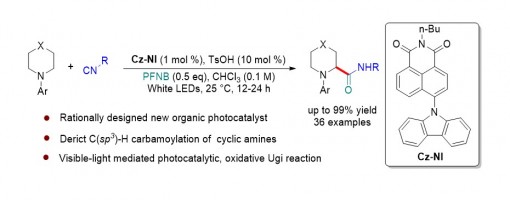
Ming-Jun Yi,§ Huan-Xin Zhang,§ Teng-Fei Xiao, Ji-Hua Zhang, Zhi-Tao Feng, Li-Pu Wei, Guo-Qiang Xu*, and Peng-Fei Xu*
https://doi.org/10.1021/acscatal.1c00242
Abstruct
Herein, we report a general strategy for the rational design of an efficient organic photocatalyst, and we describe a robust method for the direct C(sp3)–H carbamoylation of saturated aza-heterocycles under mild conditions by using a naphthalimide (NI)-based organic photocatalyst. This protocol provides a concise and practical approach for the rapid installation of a valuable amide bond onto pharmaceutically useful saturated aza-heterocycles to access a wide range of cyclic α-amino amides. A series of mechanism investigations have demonstrated this transformation undergoes a visible-light photocatalytic, oxidative Ugi reaction process.
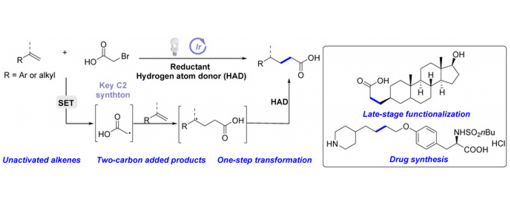
Jie Fang, Qiang Hu, Wan-Li Dong, Guo-Qiang Xu, Xiu-Qin Hu, Yong-Chun Luo, and Peng-Fei Xu
https://doi.org/10.31635/ccschem.020.202000542
Abstract
Direct introduction of a carboxyl group into molecules is one of the most useful methods for the preparation of carboxylic acids, which avoids the conversions of various pre-existing functional groups and features good step- and atom-economy. However, the methods for the direct synthesis of two-carbon added carboxylic acids from the precursors remain rare. Herein, we first report a general and mild method for the direct synthesis of a range of aliphatic acids by photoredox catalyzed hydrocarboxymethylation of alkenes with good toleration of various functional groups, in which bromoacetic acid is utilized as an ideal two-carbon synthon. The synthetic utility of this hydrocarboxymethylation protocol is further demonstrated by the concise synthesis of two marketed drugs, sensipar and tirofiban, from commercially available starting materials.
Selective Decarbonylation via Transition-Metal-Catalyzed Carbon–Carbon Bond Cleavage
Hong Lu, Tian-Yang Yu, Peng-Fei Xu, Hao Wei*
https://dx.doi.org/10.1021/acs.chemrev.0c00153
Regiodivergent Access to 2- or 3- Substituted Indanones: Catalyst-Controlled Carboacylation via C–C Bond Activation
Pengcheng Shao,§ Tianyang Yu,§ Hong Lu, Peng-Fei Xu and Hao Wei,*
https://doi.org/10.31635/ccschem.020.202000448

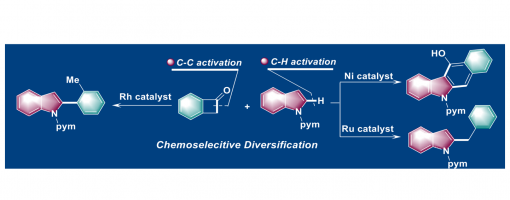
Divergent Coupling of Benzocyclobutenones with Indoles via C−H and C−C Activations
Hong Lu+, Tian-Tian Zhao+, Jin-Hua Bai, Dan Ye, Peng-Fei Xu, and Hao Wei*
https://onlinelibrary.wiley.com/doi/10.1002/anie.202010244
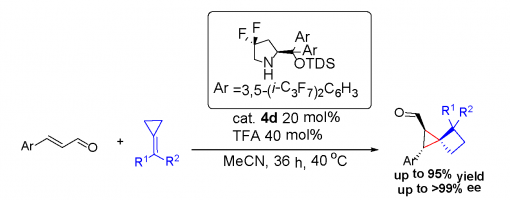
Highly Enantioselective Construction of Strained Spiro[2,3]hexanes through a Michael Addition/Ring Expansion/ Cyclization Cascade
Chuan-Gang Zhao, Zhi-Tao Feng, Guo-Qiang Xu,* Ang Gao, Jing-Wei Chen, Zhu-Yin Wang, and Peng-Fei Xu*
https://doi.org/10.1002/anie.201912834
Enantioselective Synthesis of Spirorhodanine-Pyran Derivatives via Organocatalytic [3 + 3] Annulation Reactions between Pyrazolones and Rhodanine-Derived Ketoesters
Dong-Sheng Ji, Yong-Chun Luo, Xiu-Qin Hu, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.orglett.9b04571

A lutidine-promoted photoredox catalytic atom-transfer radical cyclization reaction for the synthesis of 4-bromo-3,3-dialkyl-octahydroindol-2-ones
Quan-Sheng Zhao, Guo-Qiang Xu, Ji-Tao Xu, Zhu-Yin Wang and Peng-Fei Xu*
https://doi.org/10.1039/C9CC09876C

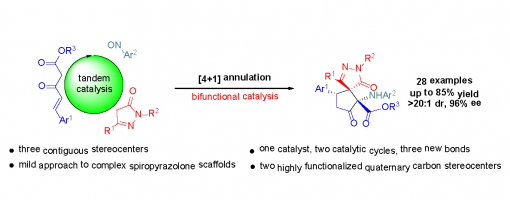
Asymmetric Organocatalytic [4 + 1] Annulations Involving a Polarity Reversal Process: A Tandem Catalytic Approach to Highly Functionalized Spiropyrazolone Derivatives
Chang-Yin Tan, Hong Lu, Jia-Lu Zhang, Jin-Yu Liu, and Peng-Fei Xu*
https://doi.org/10.1021/acs.joc.9b02684
Ni-Catalyzed 1,2-Acyl Migration Reactions Triggered by C−C Bond Activation of Ketones
Cheng Jiang, Hong Lu, Wen-Hua Xu, Jianing Wu, Tian-Yang Yu, Peng-Fei Xu, and Hao Wei*
https://pubs.acs.org/doi/10.1021/acscatal.9b04112

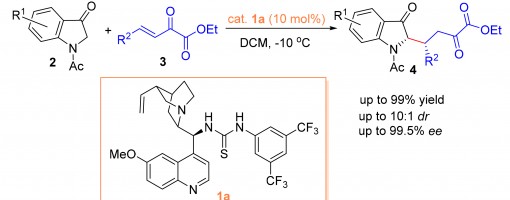
Bifunctional Thioureas Catalyzed Asymmetric Michael Addition of 1-Acetylindolin-3-ones to β,γ-Unsaturated α-Keto Esters
Liu Yaozong, Xu Pengfei, Ma Jianjun, Li Xiaoming, Liang Ruiyuan, Teng Zhijun
https://doi.org/10.6023/cjoc201911027
Abstract
The asymmetric Michael addition of 1-acetylindolin-3-ones to β,γ-unsaturated α-keto esters catalyzed by bifunctional thioureas has been developed. Enantio-pure 2-substituted indolin-3-one derivatives were obtained easily in excellent yields (up to 99%) with good diastereoselectivity (up to 10∶ 1) and excellent enantioselectivities (up to 99.5%), which would be useful for the chiral synthesis of indole-related compounds.
Dehydrogenative Silylation of Alkenes for the Synthesis of Substituted Allylsilanes via Photoredox, HAT, and Cobalt Catalysis
Wan-Lei Yu, Yong-Chun Luo, Lei Yan, Dan Liu, Zhu-Yin Wang and Peng-Fei Xu*
https://doi.org/10.1002/anie.201904707

Photocatalyzed Metal-Free Alkylheteroarylation of Unactivated Olefins via Direct Acidic C(sp3)–H Bond Activation
Jie Fang, Wan-Li Dong, Guo-Qiang Xu and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.9b01329

Metal-Free Visible-Light-Induced Construction of Difluoro-Containing Dibenzazepines
Dan Liu, Meng-Jie Jiao, Xing-Zhi Wang, and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.9b01629

Aroylchlorination of 1,6-Dienes via a Photoredox Catalytic Atom-Transfer Radical Cyclization Process
Quan-Sheng Zhao, Guo-Qiang Xu, Hui Liang, Zhu-Yin Wang, and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.9b03222

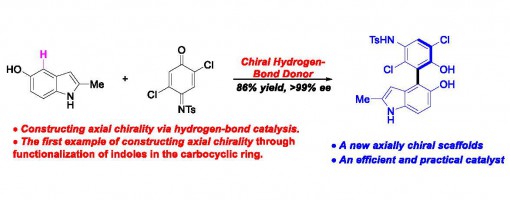
An Atropo-enantioselective Synthesis of Benzo-Linked Axially Chiral Indoles via Hydrogen-Bond Catalysis
Jin-Yu Liu, Xie-Chao Yang, Zhen Liu, Yong-Chun Luo, Hong Lu, Yu-Cheng Gu, Ran Fang,and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.9b01828
Synthesis of Indolo[2,1-a]isoquinoline Derivatives via Visible-Light-Induced Radical Cascade Cyclization Reactions
Yun-Long Wei, Jian-Qiang Chen, Bo Sun and Peng-Fei Xu *
https://doi.org/10.1039/C9CC02388G

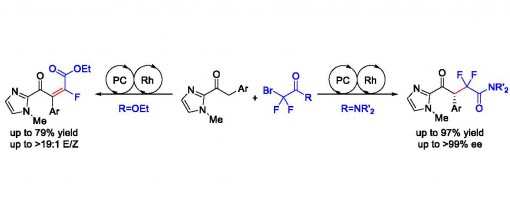
Dual Catalytic Switchable Divergent Synthesis: An Asymmetric Visible-Light Photocatalytic Approach to Fluorine-Containing γ-Keto Acid Frameworks
Hui Liang, Guo-Qiang Xu, Zhi-Tao Feng, Zhu-Yin Wang, and Peng-Fei Xu*
https://doi.org/10.1021/acs.joc.8b02316
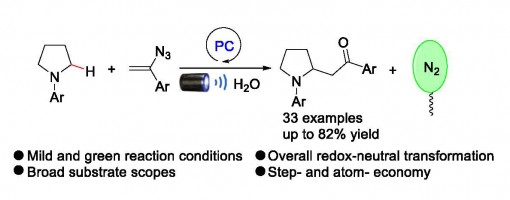
Visible Light Photoredox-Catalyzed α‑Alkylation of Cyclic Tertiary Arylamines
Ji-Tao Xu, Guo-Qiang Xu, Zhu-Yin Wang, and Peng-Fei Xu*
https://doi.org/10.1021/acs.joc.9b02347
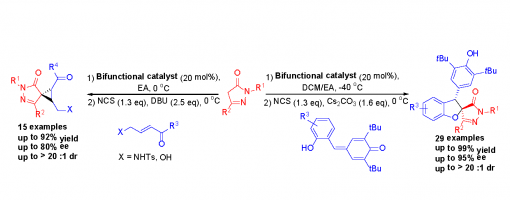
One-Pot Asymmetric Synthesis of Spiropyrazolone-Linked Cyclopropanes and Benzofurans through a General Michael Addition/Chlorination/Nucleophilic Substitution Sequence
Hong Lu,§ Huan-Xin Zhang,§ Chang-Yin Tan, Jin-Yu Liu, Hao Wei, and Peng-Fei Xu*
https://doi.org/10.1021/acs.joc.9b01454
Quaternary Carbon Center Forming [3 + 2] Cyclization Reaction by Adjusting the Substituents of Substrates
Xie-Chao Yang, Jin-Yu Liu, Zhen Liu, Xiu-Qin Hu,* and Peng-Fei Xu*
https://doi.org/10.1021/acs.joc.9b02041

Photocatalytic decarboxylative [2+2+1] annulation of 1,6-enynes with N-hydroxyphthalimide esters for the synthesis of indene-containing polycyclic compounds
Meng-Jie Jiao, Dan Liu, Xiu-Qin Hu* and Peng-Fei Xu*
https://doi.org/10.1039/C9QO01166H

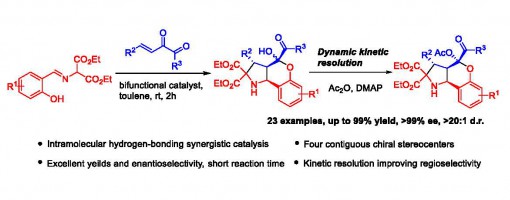
Synergistic Promotion by Intramolecular Hydrogenbonding: a Bi-functionally Catalyzed Cascade Reaction for the Synthesis of Enantiopure Chromenopyrrolidines
Jian Cao, Jin-Yu Liu, Yi-Ming Zhang, Zhu-Yin Wang and Peng-Fei Xu*
https://doi.org/10.1039/C8QO01208C
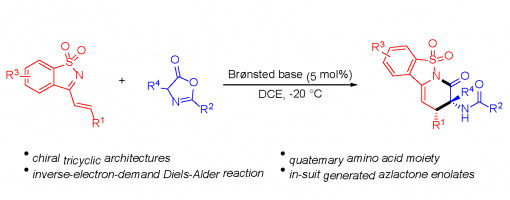
Bifunctional Brønsted Base Catalyzed Inverse Electron-Demand Aza-Diels–Alder Reactions of Saccharin-Derived 1-Azadienes with Azlactones
Xiao-Rui Ren, Jun-Bing Lin, Xiu-Qin Hu and Peng-Fei Xu *
https://doi.org/10.1039/C9QO00357F
Palladium Catalysed [3 + 2]-Annulation Reaction of Vinylcyclopropanes with Pentafulvenes: Synthesis of Polysubstituted Spiro[4,4]nona-6,8-dienes
Si-Chen Zhang, Xin-Xin Lei, Yong-Jian Yang, Yong-Chun Luo,* Huan-Huan Zhang and Peng-Fei Xu*
https://doi.org/10.1039/C9QO00467J

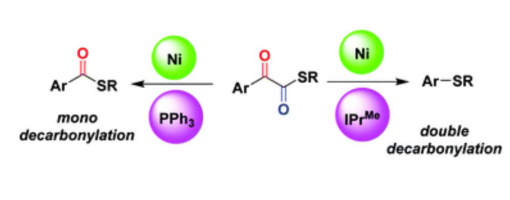
Controlled Ni-catalyzed mono- and doubledecarbonylations of a-ketothioesters
Zhao-Jing Zheng, Cheng Jiang, Peng-Cheng Shao, Wen-Fei Liu, Tian-Tian Zhao, Peng-Fei Xu* and Hao Wei*
https://doi.org/ 10.1039/C8CC09352K
Organocatalytic Asymmetric α‑Sulfenylation of 2‑Substituted Indolin-3-ones: A Strategy for the Synthesis of Chiral 2,2Disubstituted Indole-3-ones with S- and N‑Containing Heteroquaternary Carbon Stereocenter
Yong-Long Zhao,* Xing-Hai Fei, Yong-Qin Tang, Peng-Fei Xu, Fen-Fen Yang, Bin He, Xiao-Zhong Fu, Yuan-Yong Yang, Meng Zhou, Yuan-Hu Mao, Yong-Xi Dong, and Chun Li
https://doi.org/10.1021/acs.joc.9b01142

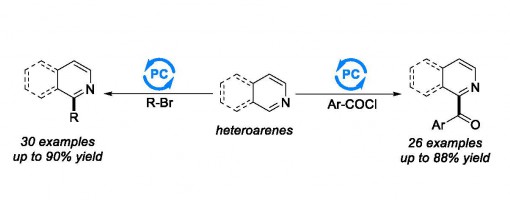
Visible Light-Mediated Direct C−H Aroylation and Alkylation of Heteroarenes
Rui Chang, Jie Fang, Jian-Qiang Chen, Dan Liu, Guo-Qiang Xu, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acsomega.9b01674
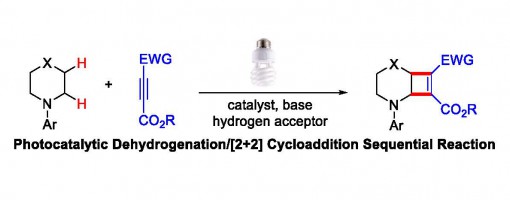
Dual C(sp3)-H Bond Functionalization of N-Heterocycles through Sequential Visible-Light Photocatalyzed Dehydrogenation/[2+2] Cycloaddition Reactions
Guo-Qiang Xu, Ji-Tao Xu, Zhi-Tao Feng, Hui Liang, Zhu-Yin Wang, Yong Qin, and Peng-Fei Xu*
https://onlinelibrary.wiley.com/doi/full/10.1002/anie.201710523
Directed Decarbonylation of Unstrained Aryl Ketones via Nickel-Catalyzed C-C Bond Cleavage
Tian-Tian Zhao, Wen-Hua Xu, Zhao-Jing Zheng, Peng-Fei Xu,* and Hao Wei*
https://pubs.acs.org/doi/10.1021/jacs.7b11591

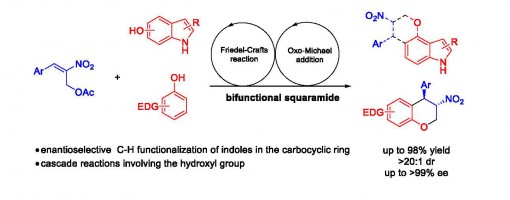
Organocatalytic, Enantioselective Friedel−Crafts Reaction of Indoles in the Carbocyclic Ring and Electron-Rich Phenols
Jin-Yu Liu, Xie-Chao Yang, Hong Lu, Yu-Cheng Gu, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.orglett.8b00503
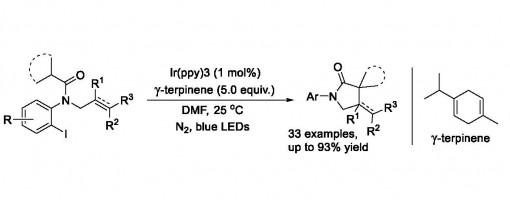
Photoredox-Induced Intramolecular 1,5‑H Transfer Reaction of Aryl Iodides for the Synthesis of Spirocyclic γ‑Lactams
Jian-Qiang Chen, Rui Chang, Jun-Bing Lin, Yong-Chun Luo, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.orglett.8b00731
Regiospecific Three-Component Aminofluorination of Olefins via Photoredox Catalysis
Jia-Nan Mo, Wan-Lei Yu, Jian-Qiang Chen, Xiu-Qin Hu,* and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.orglett.8b01760

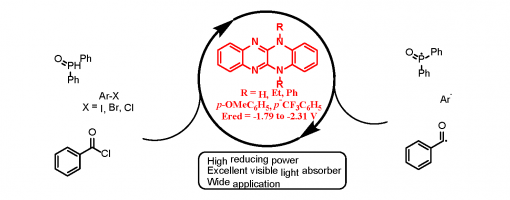
Design, Synthesis, and Application of Highly Reducing Organic Visible-Light Photocatalysts
Dan Liu, Meng-Jie Jiao, Zhi-Tao Feng, Xing-Zhi Wang, Guo-Qiang Xu, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.orglett.8b02420
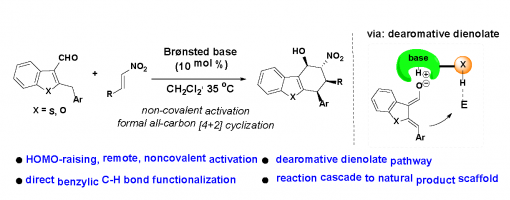
Dearomative Dienolate-Mediated Catalysis: A Remote Activation Strategy for Asymmetric Functionalization of Benzylic C−H Bonds of Heteroaryl Aldehydes
Yang Wang, Jun-Bing Lin, Ji-Kang Xie, Hong Lu, Xiu-Qin Hu,* and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.orglett.8b02523
Alkene Functionalization for the Stereospecific Synthesis of Substituted Aziridines by Visible-Light Photoredox Catalysis
Wan-Lei Yu, Jian-Qiang Chen, Yun-Long Wei, Zhu-Yin Wang and Peng-Fei Xu *
https://doi.org/10.1039/C7CC09151F

A New Approach to Access Difluoroalkylated Diarylmethanes via Visible-Light Photocatalytic Cross-Coupling Reactions
Yin-Na Zhao, Yong-Chun Luo, Zhu-Yin Wang and Peng-Fei Xu *
https://doi.org/10.1039/C8CC01486H
Synthesis of Five-Membered Cyclic Nitrones Based on the Lewis Acid-Catalysed [3+2]-Annulation Reaction of Donor–Acceptor Cyclopropanes with 1,4,2-Dioxazoles
Zhe-Hao Wang, Huan-Huan Zhang,* Peng-Fei Xu and Yong-Chun Luo*
https://doi.org/10.1039/C8CC04656E

Transition-Metal-Free Selective C-H Benzylation of Tertiary Arylamines by a Dearomatization-Aromatization Sequence
Guo-Qiang Xu, Zhi-Tao Feng, Ji-Tao Xu, Zhu-Yin Wang, Yong Qin,* and Peng-Fei Xu*
https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.201803211
Organocatalytic Regiodivergent C-C Bond Cleavage of Cyclopropenones: A Highly Efficient Cascade Approach to Enantiopure Heterocyclic Frameworks
Jian Cao, Ran Fang, Jin-Yu Liu, Hong Lu, Yong-Chun Luo, and Peng-Fei Xu*
https://chemistry-europe.onlinelibrary.wiley.com/doi/full/10.1002/chem.201803861

A C1-symmetric N-heterocyclic carbene catalyzed oxidative spiroannulation of isatin-derived enals: highly enantioselective synthesis of spirooxindole -lactones
Jun-Bing Lin, Xi-Na Cheng, Xiao-Dong Tian, Guo-Qiang Xu, Yong-Chun Luo and Peng-Fei Xu*
https://doi.org/10.1039/C8RA02009D

Palladium-Catalyzed Direct C(sp3)–H Arylation of Indole-3-ones with Aryl Halides: A Novel and Efficient Method for the Synthesis of Nucleophilic 2-Monoarylated Indole-3-ones
Yong-Long Zhao,* Yong-Qin Tang, Xing-Hai Fei, Tao Xiao, Ya-Dong Lu, Xiao-Zhong Fu, Bin He, Meng Zhou, Chun Li, Peng-Fei Xu * and Yuan-Yong Yang*
https://doi.org/10.1039/C8RA04807J
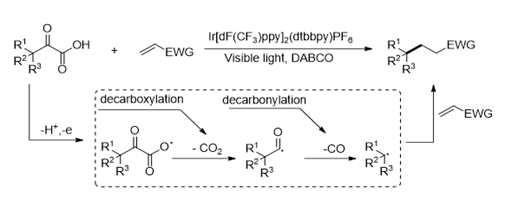
Direct Decarboxylative−Decarbonylative Alkylation of α‑Oxo Acids with Electrophilic Olefins via Visible-Light Photoredox Catalysis
Jian-Qiang Chen, Rui Chang, Yun-Long Wei, Jia-Nan Mo, Zhu-Yin Wang, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.joc.7b02628
Participation of β‑Ketothioamides in N‑Heterocyclic Carbene- Catalyzed [3 + 3] Spiroannulation: Asymmetric Synthesis of Functionalized Spiro-piperidinone Derivatives
Hong Lu, Chang-Yin Tan, Huan-Xin Zhang, Jia-Lu Zhang, Jin-Yu Liu, Hong-Yu Li, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.joc.8b02520

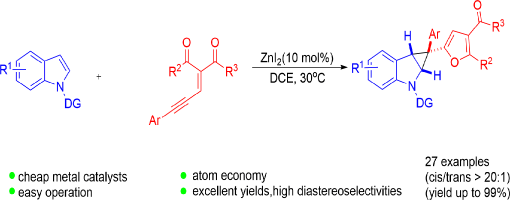
Directing Group Assisted ZnI2-Catalyzed Cyclopropanation of Indoles via 2-Furylcarbenoids
Wei Chen, Dong-Sheng Ji, Yong-Chun Luo,* Zhu-Yin Wang and Peng-Fei Xu *
https://doi.org/10.1039/C8QO00232K
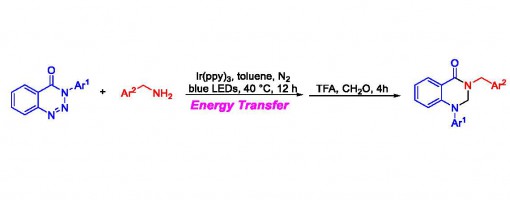
Synthesis of Quinazolinone Derivatives via a Visible-Light Photocatalyzed Denitrogenation Rearrangement Process
Chen-Guang Li, Guo-Qiang Xu, Peng-Fei Xu*
https://doi.org/10.1016/j.jphotochem.2017.12.004
(Diacetoxyiodo)benzene-mediated Selective Synthesis of α-Azido Ketones or Acyl Azides from β-Keto Acids
Zhao-Jing Zheng, Tian-Yang Yu, Peng-Fei Xu* and Hao Wei*
https://doi.org/10.1002/ajoc.201800319

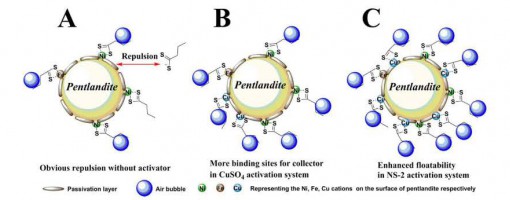
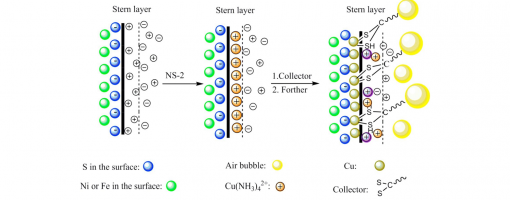
Investigation on the Promoted Flotation Behavior of Pentlandite by Ammoniacal Copper(II) Solution
Jian Cao, Li Qi, Xiao-Dong Tian, Jun-Jun Jia, Xiu-Qin Hu, Yong-Chun Luo, Peng-Fei Xu*
https://doi.org/10.1016/j.colsurfa.2018.02.057
Effect of Ammonia Molecules on the Separation of Pentlandite from Serpentine Using Copper (II) as Activator
Yun Bao, Guoqiang Xu, Xiaodong Tian, Pengfei Xu,* Jiantai Ma*
https://doi.org/10.1016/j.seppur.2018.02.021

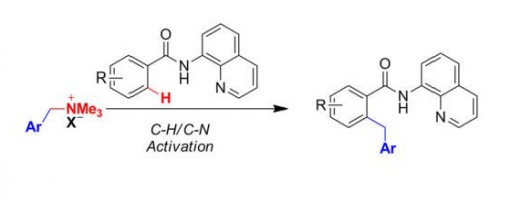
Nickel-Catalyzed Directed Benzylation of Ortho C-H Bonds in Aromatic Amides through C-H/C-N Cleavage
Jiawei Li,Zhaojing Zheng,Tiantian Xiao,Peng-Fei Xu,and Hao Wei*
https://doi.org/10.1002/ajoc.201700569
Rh(II) Catalyzed High Order Cycloadditions of 8-Azaheptafulvenes with N-Sulfonyl 1,2,3-Triazloes or α-Oxo Diazocompounds
Wei Chen, Ya-Li Bai, Yong-Chun Luo* and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.6b03542

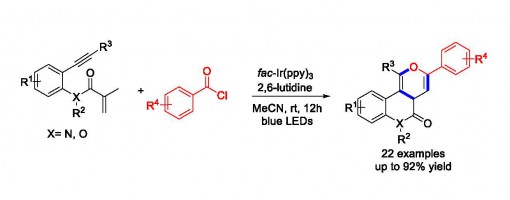
Synthesis of Fused Pyran Derivatives via Visible-Light-Induced Cascade Cyclization of 1,7-Enynes with Acyl Chlorides
Chen-Guang Li, Guo-Qiang Xu, and Peng-Fei Xu*
https://pubs.acs.org/doi/10.1021/acs.orglett.6b03684
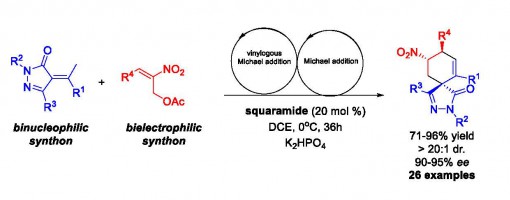
Quaternary Carbon Center Forming Formal [3 + 3] Cycloaddition Reaction via Bifunctional Catalysis: Asymmetric Synthesis of Spirocyclohexene Pyrazolones
Jin-Yu Liu, Jing Zhao, Jia-Lu Zhang, and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.7b00610
Highly Enantioselective Construction of Hajos−Wiechert Ketone Skeletons via an Organocatalytic Vinylogous Michael/Stetter Relay Sequence
Zhi-Long Jia, Yao Wang, Chuan-Gang Zhao, Xiao-Hai Zhang, and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.7b00767

Visible-Light-Induced Aza-Pinacol Rearrangement: Ring Expansion of Alkylidenecyclopropanes
Wen-Deng Liu, Guo-Qiang Xu, Xiu-Qin Hu, and Peng-Fei Xu*
https://doi.org/10.1021/acs.orglett.7b02989
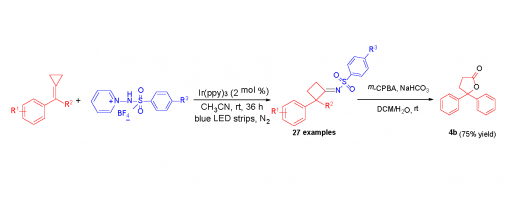
3,4,5-Trimethylphenol and Lewis Acid Dual-Catalyzed Cascade Ring-Opening/Cyclization: Direct Synthesis of Naphthalenes
He Ma, Xiu-Qin Hu,* Yong-Chun Luo, and Peng-Fei Xu*
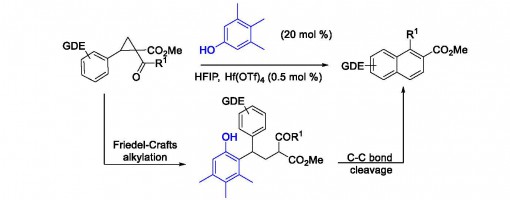
https://doi.org/10.1021/acs.orglett.7b03392
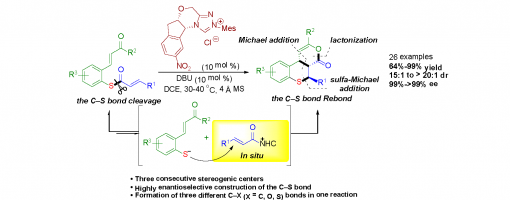
N-Heterocyclic Carbene-Catalyzed Atom-Economical and Enantioselective Construction of the C−S Bond: Asymmetric Synthesis of Functionalized Thiochromans
Hong Lu, Jia-Lu Zhang, Jin-Yu Liu, Hong-Yu Li, and Peng-Fei Xu*,
https://doi.org/10.1021/acscatal.7b02651
Kinetic Resolution via Supramolecular Iminium Catalysis: Multiactivation Enables the Asymmetric Synthesis of β-Aryl Substituted Aldehydes and Densely Functionalized Cyclohexanes
Zhi-Long Jia, Yao Wang,* Guo-Qiang Xu and Peng-Fei Xu*
https://doi.org/10.1039/C7CC01625E